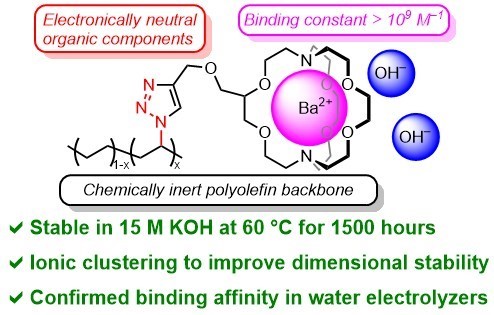
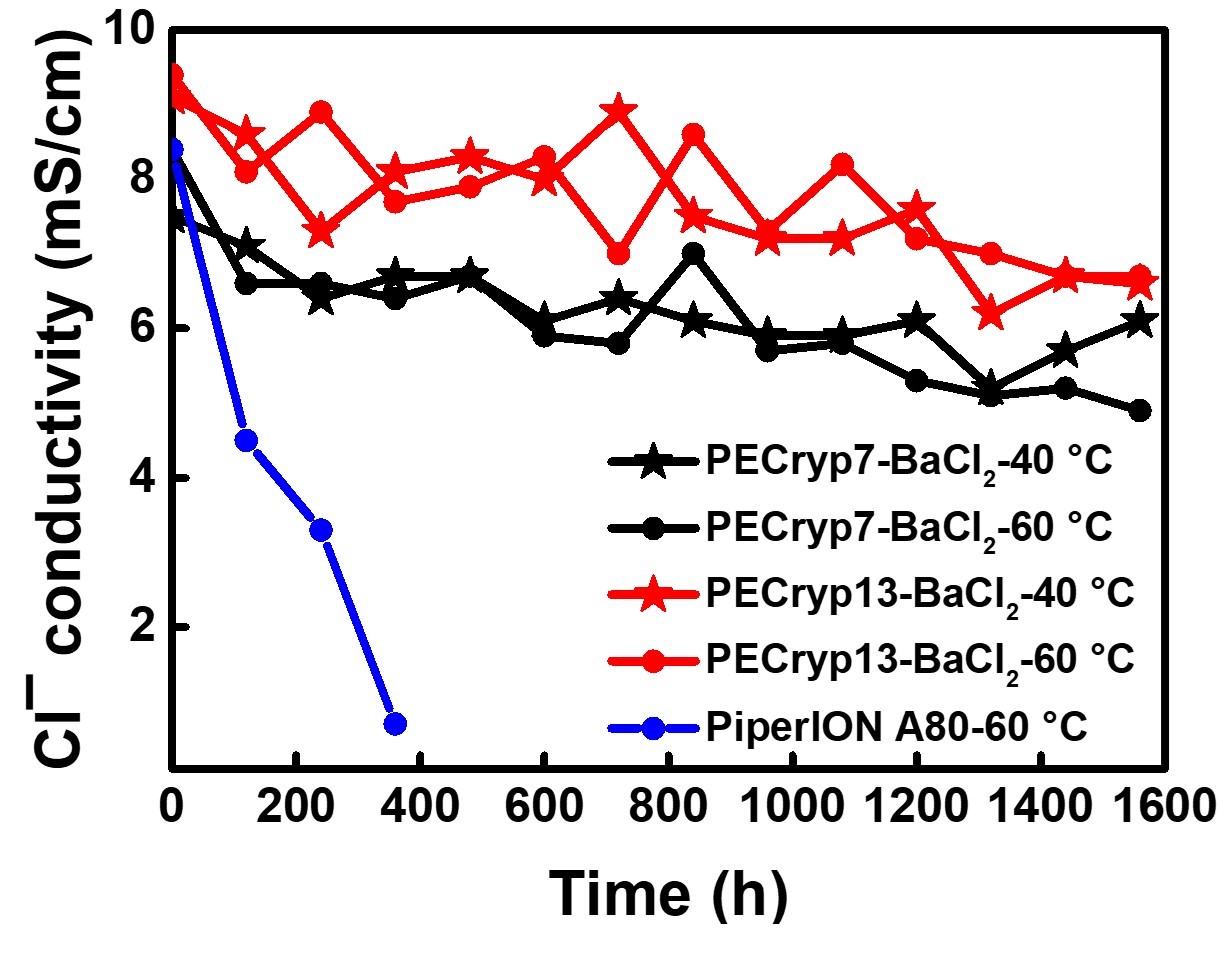
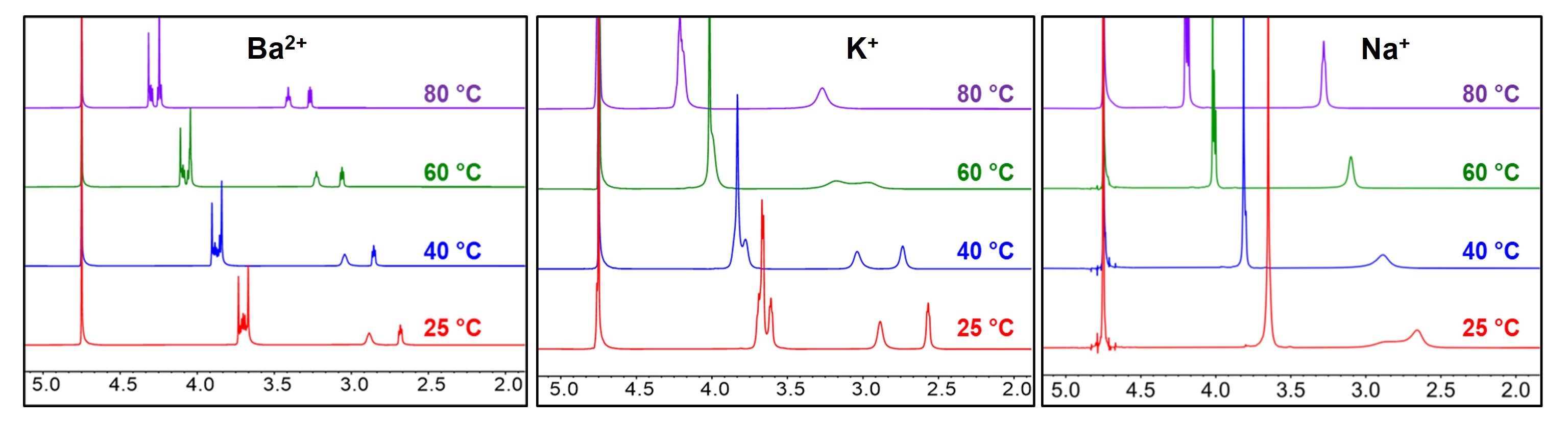
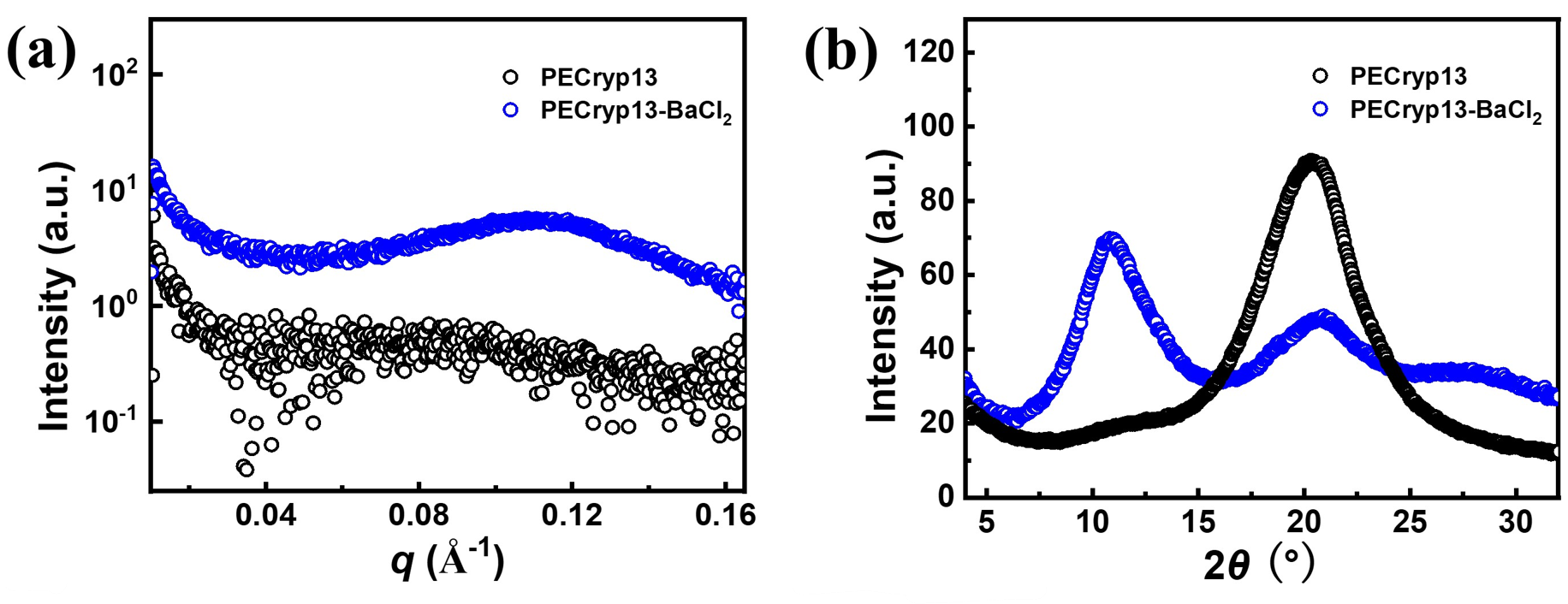
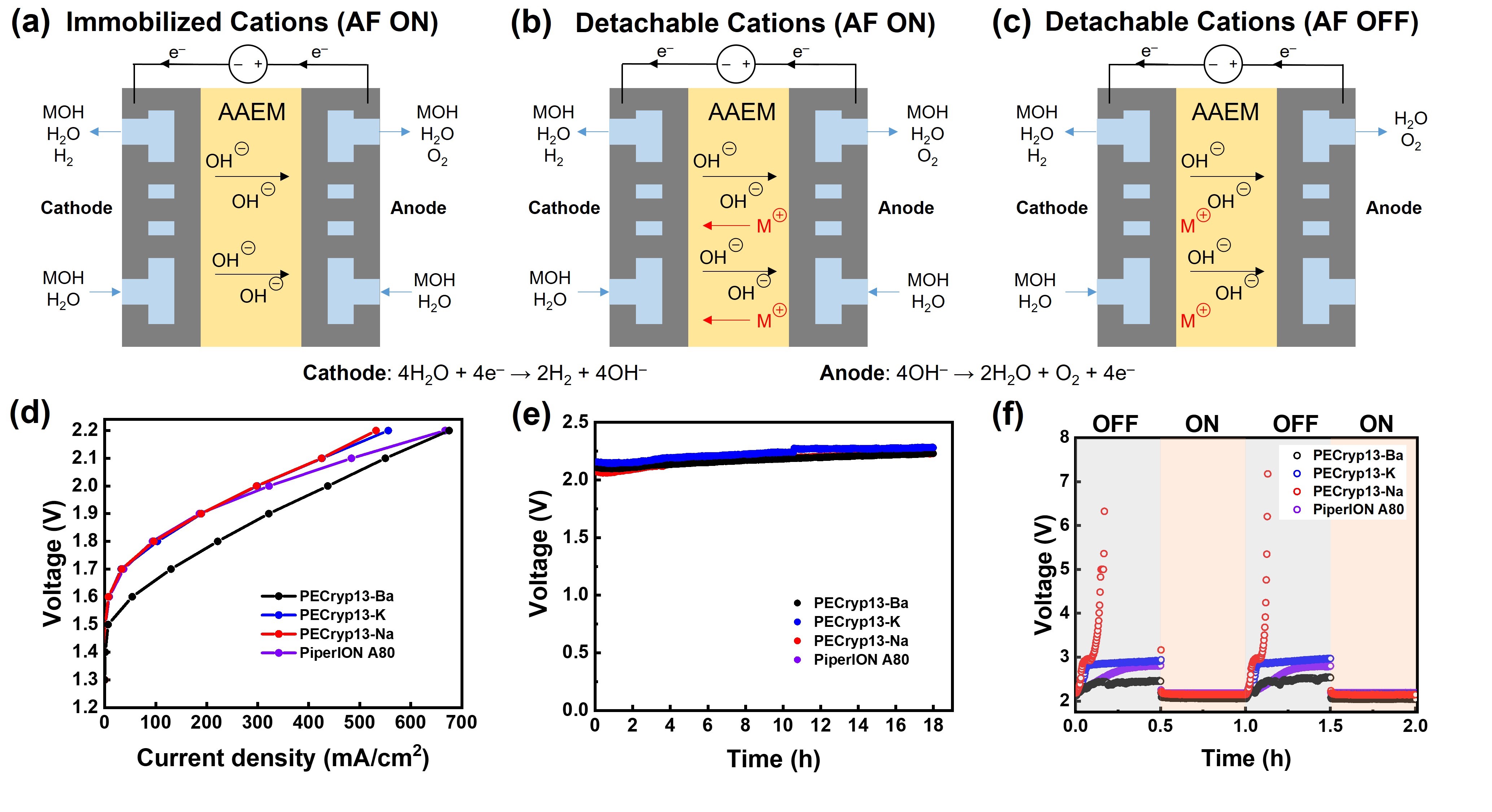
The stability of cationic functional groups is one of the key factors determining lifetime of alkaline anion-exchange membranes (AAEMs) and the AAEM-based electrochemical devices. Main-group metal and crown ether complexes are stable cations due to the absence of degradation pathways including nucleophilic substitution, Hofmann elimination, and cation redox. However, the binding strength, a key feature for AAEM applications, is overlooked in previous work. We herein propose the use of barium [2.2.2]cryptate ([Cryp-Ba]2+) as a new cationic functional group for AAEMs due to its extremely strong binding (109.5 M−1 in water at 25 °C). The [Cryp-Ba]2+-AAEMs with polyolefin backbones remain stable after treatment in 15 M KOH at 60 °C for over 1500 hours. More importantly, these AAEMs are successfully applied in water electrolyzers, and an anolyte-feeding switch method is designed to further reveal the influence of binding constants.
This research work entitled “Alkaline-stable anion-exchange membranes with barium [2.2.2]cryptate cations: the importance of high binding constants” has been published in Angewandte Chemie International Edition.
Link to the article: https://doi.org/10.1002/anie.202217742
Co-corresponding authors: Assistant Professor Yu Wang (ywang507@iccas.ac.cn), Professor Wenjuan Zhang (zhangwj@bift.edu.cn), and Professor Wei You (weiyou@iccas.ac.cn).
downloadFile